The important alloys of copper and tin from an industrial
point of view are the bronzes comprised within certain limits
of tin content. As in the case of the brasses, the addition
of tin to cooper results in the formation of a series of
solid solutions. The constitutional diagram of copper-tin
alloys is very complex, but that part of it which deals with
alloys of industrial importance is reproduced in Fig. 1.
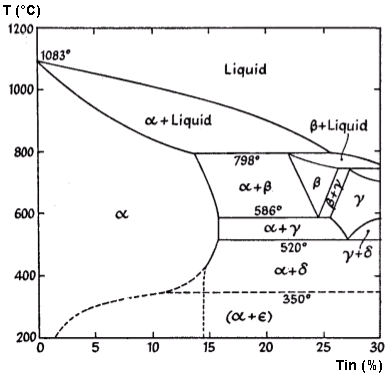
Figure 1. Constitutional Diagram of the Copper-Tin Alloys
The addition of tin to copper results in the formation of a
series of solid solutions which, in accordance with usual
practice, are referred to in order of diminishing copper
content as the α, β, γ, etc., constituents.
The diagram may be summarized as follows:
|
Percentage composition
|
Constituent just below the freezing point
|
Constituent after slow cooling to 400°C
|
|
Copper
|
Tin
|
|
100 to 87
|
0 to 13
|
α
|
α
|
|
87 to 86
|
13 to 14
|
α + β
|
α
|
|
86 to 78
|
14 to 22
|
α + β
|
α + δ
|
|
78 to 74
|
22 to 26
|
β–>(α + β)
|
α + δ
|
Further changes on cooling from 400°C to room temperature
are so sluggish that they only occur in conditions very far
removed from actual practice.
The α solution is the softest of the
constituents; it may be rolled or stamped cold, but it
hardens under this treatment much more rapidly decreases
than α-brass.
The β and a constituents do not exist in the alloy
slowly cooled to room temperature: this is due to successive
changes occurring at 586°C and 520°C whereby β is
resolved into α +γ and γ into α +
δ.
The δ constituent has the crystal structure of
γ-brass. It has a narrow range of composition
corresponding approximately to the formula Cu3lSn8
and, like all intermetallic compounds, is extremely hard and
brittle. The δ -> (α + ε) change at
350°C does not occur in commercial practice, though alloys
richer in tin may contain the a constituent, which
corresponds to Cu3Sn, and the η solid solution,
which approximates to the composition CuSn.
95:5 Copper-Tin Alloy
On cooling from the liquid condition, the solid solution
which first forms contains only about 2 percent of tin. Thus
the cast metal has a cored structure and the coring is very
marked because of the long range between liquidus and solidus;
but it may be eliminated by diffusion on cooling more slowly
or by annealing.
Any absorption of oxygen occurring during manufacture results
in the presence of SnO2 in the alloy, tending to
make it brittle. A deoxidizer such as zinc is therefore
frequently added. The addition of zinc, as in coinage bronze,
causes no change in the microscopical appearance of the
homogeneous α constituent. The zinc, however, exerts
its deoxidizing effect in the liquid, and slight hardening
effect on the solid solution. The structure of a bronze coin
shows marked deformation of the crystals. On annealing,
recrystallization takes place with subsequent crystal growth.
Twinning is a characteristic feature of the cold-worked and
annealed alloy.
90:10 Copper-Tin Alloy
This is typical gun-metal, most varieties of which, however,
contain a deoxidizer, frequently zinc (e.g. Admiralty
gun-metal, copper 88%, tin 10%, zinc 2%). The structure of
the cast material depends on the rate of cooling, both
through the range of solidification and below.
On account of the wide solidification range of the alloy and
the slow rate of tin diffusion, the apparent solubility limit
of the α solution is well below that shown in the
diagram. The cast structure is always definitely dendritic
and if coring is pronounced, some β solution may be
formed at 798°C This interdendritic β, on cooling,
gives rise to the hard δ constituent. On the other hand,
after slow cooling or prolonged annealing, the
homogeneous α constituent may be produced. A chill-cast
gun-metal will therefore be very different in structure and
properties from one which has been annealed.
85:15 Copper-Tin Alloy
This chemical composition is typical for a number of bronzes
used as bearing metals, most of which, however, contain a
little zinc as a deoxidizer. It is also the approximate
composition of bell metal.
Immediately after solidification the alloy consists of
the α and β constituents. If rapidly cooled,
these are preserved. If slowly cooled, the β (or γ)
is completely broken down below 520°C into a
complex α + δ. The α + β structure is
being replaced by α + (α + δ) complex in
the slowly cooled alloy. This accounts for the fact that sand
castings of this alloy are much harder than chill castings.
It also provides the basis of heat treatment method, applied
in the one case to bells and in the other to bearing metals.
List of Articles - Knowledge Base