The purpose of annealing may involve one or more of the following
aims:
- To soften the steel and to improve machinability.
- To relieve internal stresses induced by some previous treatment (rolling, forging,
uneven cooling).
- To remove coarseness of grain.
The treatment is applied to forgings, cold-worked sheets and wire, and
castings. The operation consists of:
- heating the steel to a certain temperature,
- "soaking" at this temperature for a time sufficient to allow the necessary changes to occur,
- cooling at a predetermined rate.
Sub-critical Anneal
It is not always necessary to heat the steel into the critical range.
Mild steel products which have to be repeatedly cold worked in the
processes of manufacture are softened by annealing at 500° to 650°C for
several hours. This is known as "process" or "close" annealing, and is
commonly employed for wire and sheets. The recrystallisation temperature
of pure iron is in the region of 500°C consequently the higher temperature
of 650°C brings about rapid recrystallisation of the distorted ferrite
Since mild steel contains only a small volume of strained pearlite a high
degree of softening is induced. As shown, Fig. 1b illustrates the
structure formed consisting of the polyhedral ferrite with elongated
pearlite (see also Fig. 2).
Prolonged annealing induces greater ductility at the expense of
strength, owing to the tendency of the cementite in the strained pearlite
to "ball-up" or spheroidise, as illustrated in Fig. 1c. This is known as
"divorced pearlite". The ferrite grains also become larger, particularly
if the metal has been cold worked a critical amount. A serious
embrittlement sometimes arises after prolonged treatment owing to the
formation of cementitic films at the ferrite boundaries. With severe
forming operations, cracks are liable to start at these cementite
membranes.
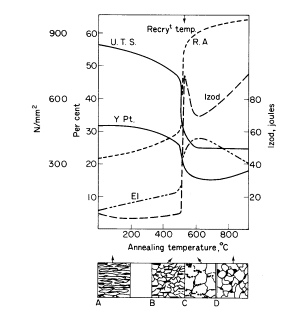
Figure 1. Effect of annealing cold-worked mild steel

Figure 2. Effect of annealing at 650°C on worked steel. Ferrite
recrystallised. Pearlite remains elongated (x600)
The modern tendency is to use batch or continuous annealing furnaces
with an inert purging gas. Batch annealing usually consists of 24-30 hrs
670°C, soak 12 hrs, slow cool 4-5 days. Open coil annealing consists in
recoiling loosely with controlled space between wraps and it reduces
stickers and discoloration. Continuous annealing is used for thin strip
(85% Red) running at about 400 m/min. The cycle is approximately up to
660°C 20 sec, soak and cool 30-40 sec. There is little chance for grain
growth and it produces harder and stiffer strip; useful for cans and
panelling.
"Double reduced" steel is formed by heavy reduction (~50%) after
annealing but it suffers from directionality. This can be eliminated by
heating between 700-920°C and rapidly quenching.
Full Anneal and Normalising Treatments
For steels with less than 0,9% carbon both treatments consist in
heating to about 25-50°C above the upper critical point indicated by the
Fe-Fe3C equilibrium diagram (Fig. 3). For higher carbon steels the
temperature is 50°C above the lower critical point.
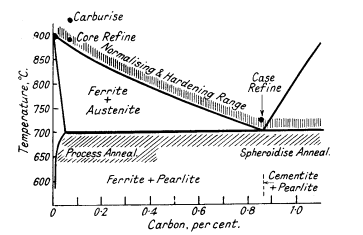
Figure 3. Heat-treatment ranges of steels
Average annealing and hardening temperatures
are:
| Carbon, % |
0.1 |
0.2 |
0.3 |
0.5 |
0.7 |
0.9 to 1.3 |
| Avg.temp. °C |
910 |
860 |
830 |
810 |
770 |
760 |
These temperatures allow for the effects of slight variations in the
impurities present and also the thermal lag associated with the critical
changes. After soaking at the temperature for a time dependent on the
thickness of the article, the steel is very slowly cooled. This
treatment is known as full annealing, and is used for removing
strains from forgings and castings, improving machinability and also when
softening and refinement of structure are both required.
Normalising differs from the full annealing in that the metal
is allowed to cool in still air. The structure and properties
produced, however, varying with the thickness of metal treated. The
tensile strength, yield point, reduction of area and impact value are
higher than the figures obtained by annealing.
Changes on Annealing
Consider the heating of a 0,3% carbon steel. At the lower critical
point (Ac1) each "grain" of pearlite changes to several minute austenite
crystals and as the temperature is raised the excess ferrite is dissolved,
finally disappearing at the upper critical point (Ac3), still with the
production of fine austenite crystals. Time is necessary for the carbon to
become uniformly distributed in this austenite. The properties obtained
subsequently depend on the coarseness of the pearlite and ferrite and
their relative distribution. These depend on:
a) the size of the austenite grains; the smaller their size the better
the distribution of the ferrite and pearlite.
b) the rate of cooling
through the critical range, which affects both the ferrite and the
pearlite.
As the temperature is raised above Ac3 the crystals increase in size.
On a certain temperature the growth, which is rapid at first, diminishes.
Treatment just above the upper critical point should be aimed at, since
the austenite crystals are then small.
By cooling slowly through the critical range, ferrite commences to
deposit on a few nuclei at the austenite boundaries. Large rounded ferrite
crystals are formed, evenly distributed among the relatively coarse
pearlite. With a higher rate of cooling, many ferrite crystals are formed
at the austenite boundaries and a network structure of small ferrite
crystals is produced with fine pearlite in the centre.
Overheated, Burnt and Underannealed Structures
When the steel is heated well above the upper critical temperature
large austenite crystals form. Slow cooling gives rise to the
Widmanstätten type of structure, with its characteristic lack of both
ductility and resistance to shock. This is known as an overheated
structure, and it can be refined by reheating the steel to just above the
upper critical point. Surface decarburisation usually occurs during the
overheating.
During the Second World War,
aircraft engine makers were troubled with overheating (above 1250°C) in
drop-stampings made from alloy steels. In the hardened and tempered condition the fractured
surface shows dull facets. The minimum overheating temperature depends on the
"purity" of the steel and is substantially lower in general
for electric steel than for open-hearth steel. The overheated structure in these
alloy steels occurs when they are cooled at an intermediate rate from
the high temperature. At faster or slower rates the overheated
structure may be eliminated. This, together with the fact that the
overheating temperature is significantly raised in the presence of high
contents of MnS and inclusions, suggests that this overheating is conected in
some way with a diffusion and precipitation process, involving MnS. This type of overheating
can occur in an atmosphere free from oxygen, thus emphasising the difference
between overheating and burning.
As the steel approaches the solidus temperature,
incipient fusion and oxidation take place at the grain boundaries. Such a
steel is said to be burnt and it is characterised by the presence of
brittle iron oxide films, which render the steel unfit for service, except
as scrap for remelting.
List of Articles - Knowledge Base