Flake graphite iron finds use due to:
- its cheapness and ease of machining;
- low-melting temperature (1140-1200°C);
- ability to take good casting impressions;
- wear resistance;
- high damping capacity;
- a reasonable tensile strength of 108-340 MPa associated with a very
high compressive strength, making it very suitable for applications
requiring rigidity and resistance to wear.
The different types vary from grey iron which is machinable to either
mottled or white iron which is not easily machinable. The white irons of
suitable composition can be annealed to give malleable cast iron.
During the last thirty years much development work has taken place and it
has been found worth while to add even expensive elements to the cheap metal
because vastly improved properties result. The new irons formed by
alloying or by special melting and casting methods are becoming competitors
to steel.
The various irons can be classified as shown in Fig. 1 based on the
form of graphite and the type of matrix structure in which it is embedded.
The metallurgical structure, composition and section of the casting
largely govern the engineering properties. One of the differences between
cast iron and steel is the presence of a large quantity of carbon,
generally 2-4%, and frequently high silicon contents. While carbon in
ordinary steel exists as cementite (Fe3C), in cast iron it occurs in two
forms:
- stable form-graphite;
- unstable form-cementite, analysed as combined carbon.
|
CAST IRON
|
|
Grey machinable iron
|
White, unmachinable iron no graphite
|
|
Flake Graphite
|
Spheroidal Graphite
|
Pearlitic
|
Martensitic
|
|
Ferritic
|
Pearlitic
|
Austenitic
|
Martensitic
|
|
|
|
Malleable iron temper carbon
graphite
|
|
|
Ferritic
|
Pearlitic
|
|
Blackheart
|
Thin Whiteheart
|
Whiteheart
|
Special Malleable
|
Figure 1. Classification of cast iron (Pearce)
Graphite is grey, soft, and occupies a large bulk, hence counteracting
shrinkage; while cementite is intensely hard, with a density of the same
order as iron. On the relative amounts, shape and the distribution of
these two forms of carbon largely depend the general properties of the
iron.
The factors mainly influencing the character of the carbon are:
- The rate of cooling.
- The chemical composition.
- The presence of nuclei of graphite and other substances.
1. Rate of cooling. A high rate of cooling
tends to prevent the formation of graphite, hence maintains the iron in a
hard, unmachinable condition. If the casting consists of varying sections
then the thin ones will cool at a much greater rate than the thick.
Consequently, the slowly cooled sections will be grey and the rapidly
cooled material will be chilled. These points are illustrated in Fig. 2,
which shows the variation in hardness of a step casting.
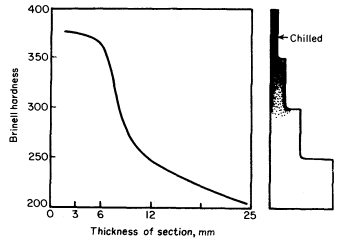
Figure 2. The relation between the rate of cooling and
hardness as indicated by sections of varying thickness
2. The effect of chemical composition.
- Carbon lowers the melting-point of the metal
and produces more graphite. Hence it favours, a soft, weak iron.
- Silicon slightly strengthens the ferrite but
raises the brittle transition temperature, Indirectly, however, it acts
as a softener by increasing the tendency of the cementite to slip up
into graphite and ferrite. Fig. 3 shows the relation between the carbon
and silicon contents in producing the different irons for one rate of
cooling. It will be noted that either a high carbon and low silicon or
low carbon and high silicon content give grey iron; the fracture can,
therefore, be misleading as to analysis, especially if the rate of
cooling is not considered. The amounts of silicon, giving the maximum
values for various properties, are also shown in Fig. 3. The percentage
of silicon is varied according to the thickness of the casting.
- Sulphur and manganese. Sulphur can exist in
iron, as either iron sulphide, FeS, or manganese sulphide, MnS. Sulphur
as FeS tends to promote cementite producing a harder iron. When
manganese is added, MnS is formed which rapidly coalesces and rises to
the top of the melt. The first effect of the manganese is, therefore, to
cause the formation of graphite due to its effect on the sulphur. The
direct effect of manganese is to harden the iron, and this it will do
when it exists in amounts greater than that required to combine with the
sulphur-1 part sulphur to 1,72 part manganese.
- Phosphorus has a little effect on the
graphite-cementite ratio; but renders the metal very fluid indirectly
through the production of a low-melting constituent, which is readily
recognised in the micro-structure (Fig. 4). In the production of sound
castings of heavy section, phosphorus should be reduced to about 0,3% in
order to avoid shrinkage porosity.
- Trace elements not normally considered in routine analyses can exert
a profound influence upon the characteristics of cast iron. Examples are
0,1% of aluminium graphitises, antimony embrittles, lead, tellurium
promotes carbide but reduces strength of iron; 0,003% of hydrogen can
greatly affect soundness of castings and tends to coarsen graphite.
Nitrogen behaves as a carbide stabiliser; oxygen has no specific effect.
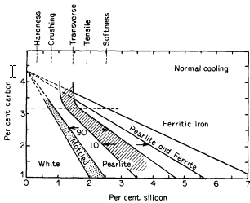
Figure 3. Diagram indicating the structures of iron resulting from variation of silicon and carbon contents
|
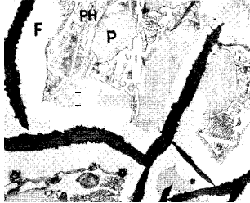
Figure 4. Common grey iron showing ferrite (F), pearlite (P)
and phosphide eutectic (PH) (x250). Ferrite is associated with the
graphite. Note banded structure in the phosphide
eutectic
|
The carbon equivalent value. From Fig. 5 it will be seen that the
eutectic E is at 4,3% carbon and irons with a greater carbon content will
(under suitable conditions) start freezing by throwing out kish graphite
of large size. With carbon contents progressively less than 4,3% normal
graphite is formed in diminishing quantities until a mottled or white iron
range is reached. Naturally other elements, especially silicon and
phosphorus, affect the composition of the eutectic point in a complex
alloy and a carbon equivalent value is suggested as an index which
converts the amount of these elements into carbon replacement values.
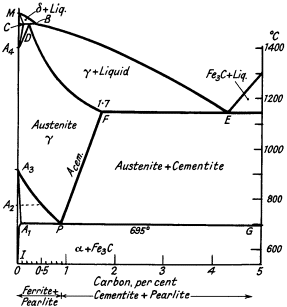
Figure 5. Iron-cementite equilibrium diagram
Carbon equivalent value (CE) = Total C% + 1/3 (Si% + P%)
For a given cooling rate the carbon equivalent value, therefore,
determines how close a given composition of iron is to the eutectic (CE
4,3) and therefore how much free graphite is likely to be present, and
consequently the probable strength in a given section: the carbon
equivalent value is also a useful guide to chilling tendency of a given
section, although it must be borne in mind that pouring temperature,
cooling rate and alloying elements have a marked influence.
List of Articles - Knowledge Base